A Philadelphia jury found that Wyeth’s hormone replacement therapy Prempro caused an Arkansas woman’s breast cancer and awarded the victim’s family $1.5 million. The jury found that Wyeth was negligent in failing to provide adequate warnings about the risk of breast cancer associated with the use of Prempro.
We expect the jury to return this week with a decision on punitive damages. In Maryland, under Owens-Illinois, Inc. v. Zenobia, 325 Md. 420 (1992), a landmark Maryland Court of Appeals’ opinion, punitive damages in this case against Wyeth for failing to warn about the risks of Prempro would have to be supported by a showing that the conduct of Wyeth was malicious, or the result of evil motive, or ill will. There are no such allegations in the Prempro litigation.
Background on Prempro
 Prempro is a hormone replacement therapy. It is not an awful medication that should be recalled. It has its place with some patients in some circumstances. The problem was that Wyeth pushed Prempro as though it could solve everything from tooth loss to macular degeneration to the common cold. (I made up the last point but you get the point.) Menopausal women were looking for solutions to so many things that come with aging were quick to buy in that hormone replacement therapy could be a panacea. But it is not.
Prempro is a hormone replacement therapy. It is not an awful medication that should be recalled. It has its place with some patients in some circumstances. The problem was that Wyeth pushed Prempro as though it could solve everything from tooth loss to macular degeneration to the common cold. (I made up the last point but you get the point.) Menopausal women were looking for solutions to so many things that come with aging were quick to buy in that hormone replacement therapy could be a panacea. But it is not.
This is not great, but it does not surprise us to learn that drug companies contend that their product cures everything. But plaintiffs’ lawyers argue about something more nefarious. They claim that the drug maker’s strategy distracted women and doctors from the link between Prempro and breast cancer. The company allegedly capitalized on the fact that women were very fearful about heart disease and Alzheimer’s Disease but did not associate these ailments conditions with menopause or estrogen loss. So they advertised to impress upon women that menopause caused estrogen loss and estrogen loss caused heart disease and dementia. The plan was that they would be so worried about these issues that they would ignore the mounting evidence of the link between breast cancer and Prempro.
The Study That Fueled This Litigation
Prempro was never the same after July 2002 when the NIH released a study called the Women’s Health Initiative Clinical Trial of Estrogen and Progestin. The study confirmed other studies that showed an increase in the incidence of breast cancer in women taking estrogen and progestin.
The results from the Women’s Health Initiative were so stunning that the study’s authors found that breast cancer risks outweighed any benefits and ended the trial. The women who received estrogen and progestin not only had a higher incidence of breast cancer, the also had cancers that were larger and at a more advanced stage than those in the placebo groups.
The study also found an increase in cardiovascular events within a few years of treatment. The irony of that is right, right? There was such a concerted effort to establish the prevention of cardiovascular disease as a sign for Prempro. Now this study was telling us that this therapy increases the risk of cardiovascular disease.
How Did the Prempro Cases End?
The Prempro cases ended with an $896 million settlement that resolved about 60% of the cases. The remaining cases resolved. The lawyers did a good bit of squabbling over the attorneys’ fees after the fact, but that has all been resolved now.
Are There Prempro Breast Cancer Cases in 2020?
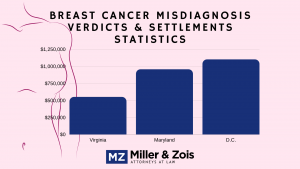
There is a breast cancer warning on Prempro now and the ridiculous marketing of hormone replacement therapy is nothing like it used to be. Our lawyers are not taking Prempro breast cancer cases in 2018 and we are unaware of any lawyers who are. Our firm is not handling these cases.
Is It Worth the Breast Cancer Risk to Take Prempro?
There is an argument being made in 2020 that the Prempro risks are overblown. Should you take Prempro? This is the wrong question to ask a lawyer, right? You should ask your doctor this question.
- Lancet is a big-time medical journal. This is what they had to say about this issue in a study in August 2019 that looks at menopausal hormone therapy and breast cancer risk. The study says that women who take hormone therapy during menopause are more likely to get breast cancer and the risk has a long shelf-life. So menopausal women need to consider all the risks and, again, your doctor is the person to be speaking with. I’m truly not qualified to even form an opinion of the risk/benefits of HRT therapy.
- Another important study if you are interested
 Maryland Injury Law Center
Maryland Injury Law Center